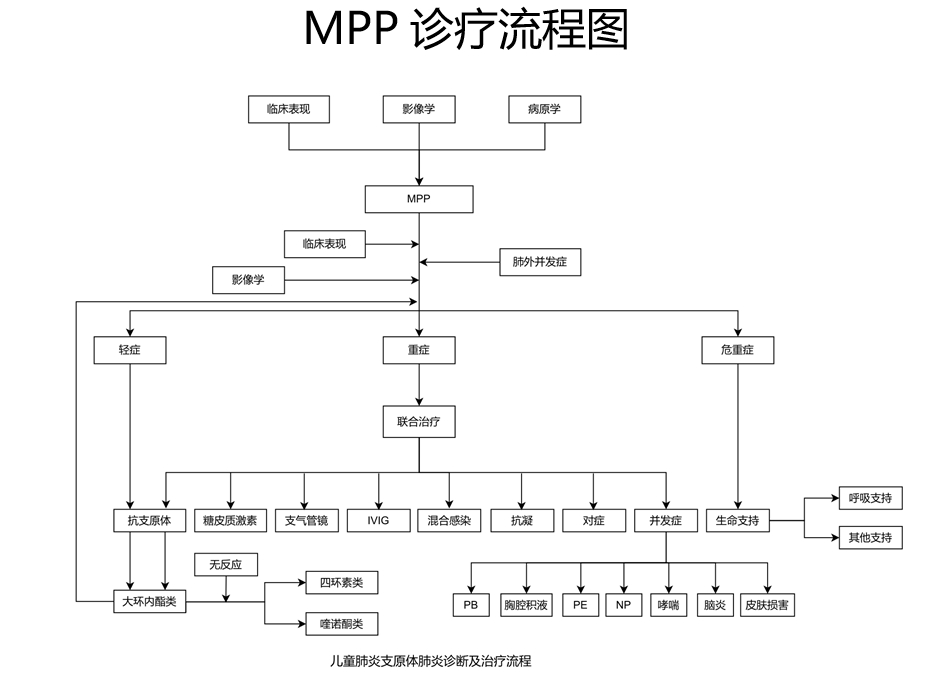
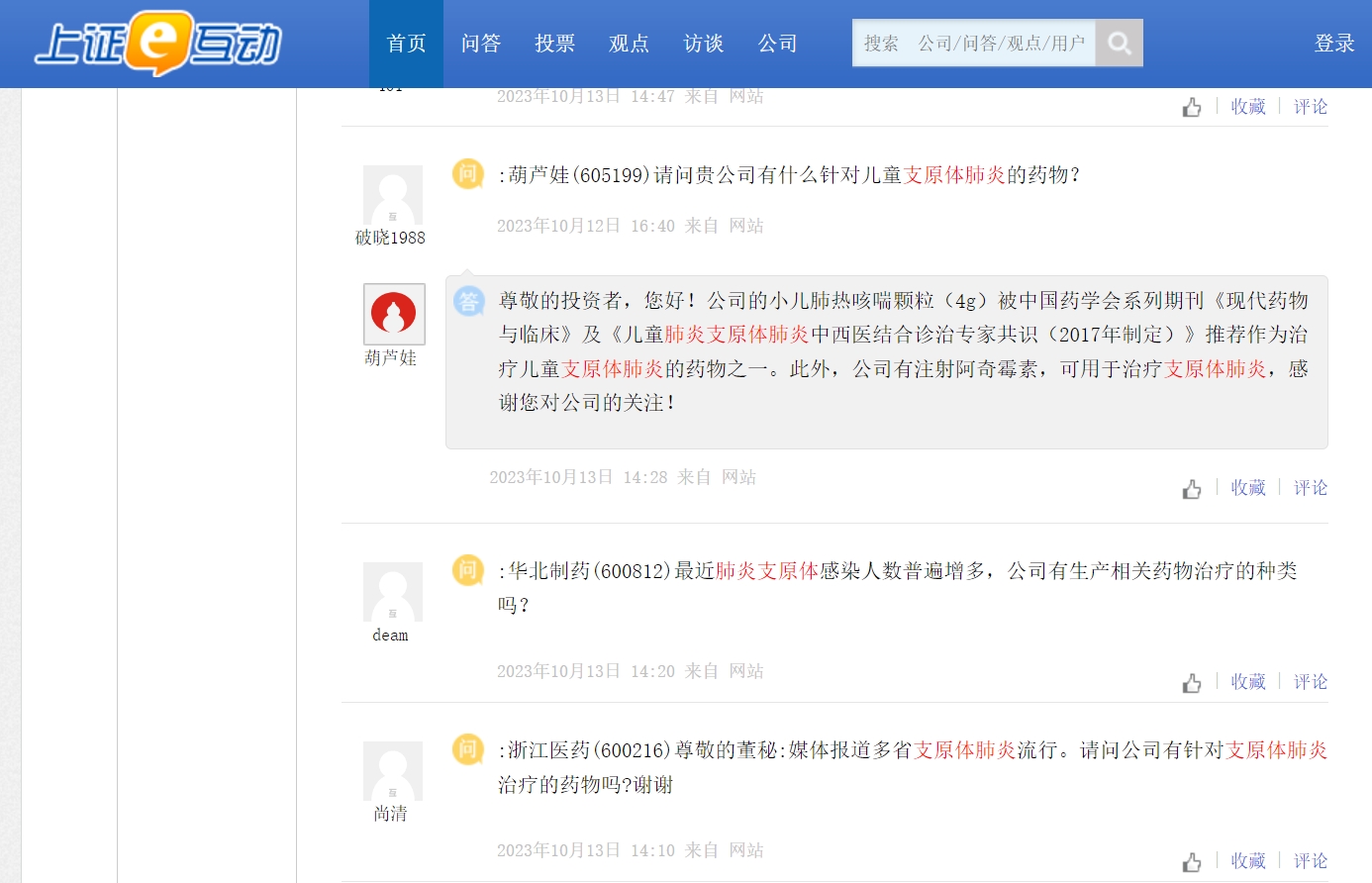
More than 20 listed companies respond to the impact of mycoplasma pneumonia
You may also be interested in:
-
More than 20 listed companies respond to the impact of mycoplasma pneumonia -
Give full play to technical advantages to help jointly build the "Belt and Road" -
The institution suggests paying attention to the allocation opportunities of livestock and poultry breeding chain -
The magic medicine is popular, and "lose weight" is competing -
Behind the "crab card scam": who sold my information -
G318 Shanghai Qingping Highway Fangting Shuiyuan Section Project was officially opened to traffic -
2023 China (Lhasa) First Digital Self driving Tour Conference -
The 24th Tangshan China Ceramic Expo opened 16
Today's Hot Spots
Recommended for you
SFC agrees to register six commodity options including staple fiber
more
-
Ensure that financial funds are used effectively -
Li Qiang Chairs the Symposium of Experts and Entrepreneurs on Economic Situation -
Go to the Canton Fair: welcome all visitors and connect with the global market -
Scale rises quarter by quarter - foreign trade imports and exports are positive -
Central Bank: RMB loans increased by 19.75 trillion yuan in the first three quarters -
The hospital with an investment of 2.8 billion yuan in Xi'an has become "yellow", and another hospital with an investment of 7 billion yuan has not yet started construction -
Married three A-share listed companies in half a month, and Middle East Capital started a new round of goods sweeping in the Chinese market -
Luzhou, Sichuan: It is proposed to implement the same maximum loan amount for the first and second housing provident fund loans
more
-
The overall recovery of the mobile phone market still takes time -
Beijing Yingfu Ruitai Real Estate Development Co., Ltd. was fined -
IMF Chief Representative in China: China remains the largest engine of global economic growth -
Senior executives come together, science and technology are brilliant and unique, focusing on the elderly - everyone insurance group is actively opening -
Guizhou Zunyi: buy houses instead of loans, encourage stronger central enterprises -
Financial knowledge is delivered to the door, and Xiamen International Bank "banks, governments and enterprises" work together to carry out finance -
Yanzhiwu · National Women's Golf Tour, full of firepower, "bowl" and beautiful finish -
The average income of 86 10 billion private equity firms in the first three quarters was 1.44%, and the quantitative performance was eye-catching
Ranking
-
Behind the "crab card scam": who sold my information -
Solution to the lack of micro pe toolbox after startup -
Leading edge hot spot: motor+algorithm technology dual drive pursuit of 618 popular products to win the first place in the platform -
On June 21, the trading limit was resumed: * ST Meigu 4-link board, Xinshida 5-day 4-link board -
Orange Cat Tea Cup Gift Box, Orange Cat Introduction_ World hotspot -
Global WeChat News: Dialogue | Go into Special Olympics and integrate into children. Special Olympics Ambassador Li Na's happiness is simple -
Cangzhou Mingzhu plans to invest 3.5 billion yuan in the construction of wet lithium diaphragm project, and the official announcement of the equity incentive plan -
These two "choked" roads... Citizen: very dangerous- World micro headlines -
Why is the price of gasoline falling fast? What is the cause of the gasoline price rise? -
Analysis of bamboo post station mode for developing small programs: realize win-win shopping with money saving!
Recent updates
-
Behind the "crab card scam": who sold my information -
Solution to the lack of micro pe toolbox after startup -
Leading edge hot spot: motor+algorithm technology dual drive pursuit of 618 popular products to win the first place in the platform -
On June 21, the trading limit was resumed: * ST Meigu 4-link board, Xinshida 5-day 4-link board -
Orange Cat Tea Cup Gift Box, Orange Cat Introduction_ World hotspot -
Global WeChat News: Dialogue | Go into Special Olympics and integrate into children. Special Olympics Ambassador Li Na's happiness is simple -
Cangzhou Mingzhu plans to invest 3.5 billion yuan in the construction of wet lithium diaphragm project, and the official announcement of the equity incentive plan -
These two "choked" roads... Citizen: very dangerous- World micro headlines -
Why is the price of gasoline falling fast? What is the cause of the gasoline price rise? -
Analysis of bamboo post station mode for developing small programs: realize win-win shopping with money saving!
this Daily news
-
The 2023 New Town Business Annual Conference of "Deep Operation Strategy" was successfully concluded -
At that time, the action plan of Guangfa Bank for the comprehensive revitalization of Northeast China was released! -
Energy Chain Smart Power and Little Red Elephant New Energy reached cooperation to jointly build a regional public charging service network -
SFC agrees to register six commodity options including staple fiber -
The average annual growth rate of the total number of business entities is 8%, and the integrated development system of market supervision in the Yangtze River Delta is accelerated -
Stable prices and continuous recovery of consumption - focus on CPI and PPI data in September -
With "the largest country" in mind and "the people's expectations" in mind, China Life Insurance Company has done a solid job for the people -
Cohesion, Jointly Build, Share, and Develop Postal Savings Bank Beijing Haidian District Branch Successfully Held Cat Garden 2023 Camp Promotion Conference -
Create safe, convenient, efficient, green and economic sustainable transportation -
Opinions on Giving Full Play to the Basic Role of People's Mediation to Promote the Governance of Complaint Sources



