New Group Standard of Silymarin Extract Released New Standard for Selecting High quality Liver Care Products

You may also be interested in:
-
The new group label of silymarin extract was released to select high-quality liver protection products -
Dalian Thermoelectric plans to purchase Kanghui New Material for 10.15 billion yuan and allocate it with new materials -
Huiyun Titanium's net profit fell 79% in the first half of the year, and it will go public in 2020 -
In the first half of the year, Fuliwang deducted 78% of the non tradable assets and raised funds in 2020 -
Penghua Zhongzheng Cloud Computing and Big Data theme increased by 24% within the year -
Xuanyuan Private Placement - Xuanyuan Kuangshi 1000 Index increased and dropped 7.63% this year -
The termination of Huatong cable will not exceed 800 million yuan of convertible bonds in 2021 -
Shanghai Pharmaceutical: controlling shareholder Shanghai Real Estate Group increased its stake in the company by 10%
Today's Hot Spots
Recommended for you
Do you feel these changes in auto insurance
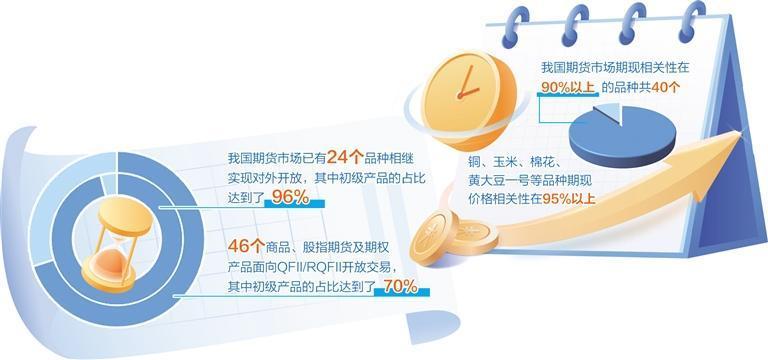
Futures market helps to build a new development pattern
CCB Suzhou builds a "warm harbor" around the people with practical actions
more
-
Bank of Communications solidly launched the "Financial Consumer Rights Protection Education and Publicity Month" activity -
The State Administration of Market Supervision: Strictly investigate and deal with violations of laws and regulations in the food safety field and effectively prevent them -
Floor washer becomes a new favorite of household appliances consumption Industry: industry concentration will increase -
Liu Weiping: Deepen international exchanges and seek common development of water resources protection -
Cao Junjie: Innovating the Regional Modern Agricultural Development Model and Accelerating the Process of Agricultural Modernization -
Xinhua Insurance fell 7.75%, net sales of institutions reached 305 million yuan -
Net purchase of 72.22 million yuan by Huaying Science and Technology trading institutions -
IT Navigation plans to acquire the equity of Aerospace New Century in cash, which is expected to generate a large amount of goodwill
more
-
New Group Standard of Silymarin Extract Released New Standard for Selecting High quality Liver Care Products -
Dalian Thermoelectric plans to purchase Kanghui New Materials for 10.15 billion yuan and raise no more than 3 billion yuan -
Huiyun Titanium's net profit in the first half of the year dropped by 79%, and the two raised funds in 2020 totaled 854 million yuan -
In the first half of the year, Fuliwang deducted 78% of non capital and raised 1.48 billion yuan in total in 2020 -
Penghua Zhongzheng Cloud Computing and Big Data theme increased by 24% within the year -
Xuanyuan Private Placement - Xuanyuan Kuangshi 1000 Index increased and dropped 7.63% this year -
The termination of Huatong cable does not exceed 800 million yuan of convertible bonds, which will be raised by 380 million yuan in 2021 -
Shanghai Pharmaceutical: controlling shareholder Shanghai Real Estate Group increased 100000 H shares of the company
Ranking
-
New Group Standard of Silymarin Extract Released New Standard for Selecting High quality Liver Care Products -
World rolling: Hangzhou, Zhejiang: energy related industries saw significant year-on-year growth in electricity consumption from January to September -
Today's view! Energy storage battery opens independent track -
Beijing Electric Power Trading Center: 2 power sales companies apply to reduce the business scope of 1 market note -
Hot focus: Meta starts the largest layoff, and more than 11000 people will be laid off -
An overseas talent war between the two giants: Tencent invested in 13 companies, and NetEase recruited 13 more big names -
Global hot: trapped in the "chop down" storm of major customers, is Goethe OK? -
Global know it all! Volvo CEO: China's new energy vehicle market has broad development space -
Volvo flagship electric SUV EX90 officially launched with lidar technology -
The staff of the flood control patrol team silently guarded the masses through the rainy night
Recent updates
-
New Group Standard of Silymarin Extract Released New Standard for Selecting High quality Liver Care Products -
World rolling: Hangzhou, Zhejiang: energy related industries saw significant year-on-year growth in electricity consumption from January to September -
Today's view! Energy storage battery opens independent track -
Beijing Electric Power Trading Center: 2 power sales companies apply to reduce the business scope of 1 market note -
Hot focus: Meta starts the largest layoff, and more than 11000 people will be laid off -
An overseas talent war between the two giants: Tencent invested in 13 companies, and NetEase recruited 13 more big names -
Global hot: trapped in the "chop down" storm of major customers, is Goethe OK? -
Global know it all! Volvo CEO: China's new energy vehicle market has broad development space -
Volvo flagship electric SUV EX90 officially launched with lidar technology -
The staff of the flood control patrol team silently guarded the masses through the rainy night
this Daily news
-
International perspective: strengthen innovative cooperation and promote the transformation and upgrading of the global automobile industry -
Do you feel these changes in auto insurance -
Futures market helps to build a new development pattern -
CCB Suzhou builds a "warm harbor" around the people with practical actions -
Bank of Communications and iFLYTEK Artificial Intelligence Joint Innovation Laboratory officially unveiled! -
The innovation of digital economy drives the creation of new growth points for urban economic development -
The Enterprise Standardization Promotion Measures will be officially implemented in 2024 -
2023 National Cyber Security Publicity Week Opens in Fuzhou -
Follow up to "Group Sailing": Close "Chain" in Win win Cooperation -
The 18th World Water Resources Conference opened in Beijing, and many experts discussed water management strategies