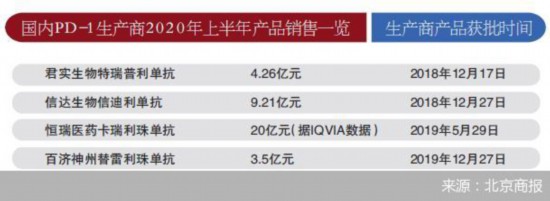
As of September 17, the semi annual reports of domestic PD-1 manufacturing pharmaceutical enterprises had been fully disclosed. The revenue of Cinda Biology, Junshi Biology and Baekje Shenzhou PD-1 medicine reached 921 million yuan, 426 million yuan and 350 million yuan respectively. Hengrui Pharmaceutical has not disclosed the sales data of PD-1 drug to the public, and the industry predicts that the sales of PD-1 drug of Hengrui Pharmaceutical will reach 2 billion yuan. Since this year, efforts to expand new indications have become the direction of major pharmaceutical enterprises. With the increasingly fierce competition in the domestic PD-1 market, more indications to seek differentiated development and more reasonable prices have become the winning points of major enterprises in the future.
Total sales exceeded 3 billion
As a new type of anti-cancer immunotherapy, PD-1/PD-L1 immunotherapy aims to make full use of the body's own immune system to resist and fight against cancer, which is different from the traditional radiotherapy and chemotherapy methods for cancer treatment. Since the first domestic PD-1 anti-cancer drug was launched in December 2018, a "4+2" competition situation has been formed in China, namely, 4 domestic drugs and 2 imported drugs.
Among the domestic PD-1 anticancer drugs, Junshi Biological has the first PD-1 drug in China. According to its 2020 semi annual report, the company's sales revenue of PD-1 drug Tereprimab was 426 million yuan. The second domestic PD-1 drug is cindilimab of Cinda Biology, and cindilimab is also the only PD-1 inhibitor successfully negotiated in the national medical insurance negotiation in 2019. According to the semi annual report in 2020, the sales volume of Cinda Biology's PD-1 inhibitor Cindelimab is 921 million yuan. According to the 2019 financial report released by Cinda Bio before, the sales volume of Cindelimab in the 10 months since its listing was 1016 million yuan. However, the sales volume of Sindhilimab in the first half of 2020 has been close to that of last 10 months, and the massive effect of medical insurance is obvious.
Karelizumab is a PD-1 drug approved for marketing by Hengrui Pharmaceutical in May 2019. In the latest semi annual report of 2020, Hengrui Pharmaceutical did not disclose the sales revenue of CARELIZAM to the public, but according to the data of IQVIA, a global medical and health service provider, the sales of CARELIZAM exceeded 2 billion yuan in the first half of the year.
In December 2019, Tirelizumab from Baekje China was approved. At the beginning of March this year, tirelizumab began to be commercialized in China. According to the financial performance of Baekje Shenzhou in the first half of 2020, the company's revenue of PD-1 drug tirelizumab is 49.95 million US dollars, about 350 million yuan.
Li Xu, an investor in the pharmaceutical industry, said in an interview with the Beijing Business Daily that Tirelizumab was not the first commercial product of Baekje China. The company had had some commercial experience before, so it could achieve a sales volume of 350 million yuan in about four months.
Accelerate the layout of new indications
Although the number of the four approved models seems small, the competition has already started. The approved indications of Cindelimab of Cinda Biology, Carelizumab of Hengrui Medicine and Tirelizumab of Baekje Shenzhou all include the treatment of patients with recurrent or refractory classical Hodgkin's lymphoma, which means that the three companies have the most direct competitive relationship.
Before the huge market, no one will give up easily. Frost Sullivan data shows that in 2030, the global market size of PD-1/PD-L1 drugs can reach 78.9 billion dollars, and the domestic market can reach 13.1 billion dollars. More indications have become a breakthrough for enterprises. In a short period of half a year, the CARELIzumab of Hengrui Medicine has successively won three major indications of liver cancer, non-small cell lung cancer and esophageal squamous cell carcinoma.
In the first half of this year, Junshi Biotechnology Terepril Monoclonal Antibody submitted a marketing application for nasopharyngeal carcinoma (the world's first new drug marketing application for anti PD-1 Monoclonal Antibody to treat recurrent/metastatic nasopharyngeal carcinoma) and urothelial cancer with two new indications, and was included in the priority review procedure. Patients with EGFR negative non-small cell lung cancer (squamous cell carcinoma and non squamous cell carcinoma) who received first-line treatment in combination with chemotherapy have been enrolled in Phase III key clinical registration. In addition, new indications for the treatment of first-line advanced squamous non-small cell lung cancer and first-line advanced non-small cell lung cancer with combination chemotherapy have been accepted successively.
The relevant person in charge of Junshi Biology said in an interview with Beijing Business Daily that the market size of PD-1 drug depends on multiple factors such as the layout of indications, the time of use of drugs for indications, and the price. Among them, the time to treat patients is the time when the indications are used. As an adjuvant treatment after surgery, the demand for first-line treatment will be greater than that for the latter, and patients will use PD-1 monoclonal antibody for a longer time. The future competition points need to consider the differentiation, innovation and service of products, and the rationality of the indication layout. The country's policy guidance, support and capital support for local start-up biopharmaceutical companies will also be the external forces affecting the future competition in the PD-1 drug market.
Can I get medical insurance
Whether it is to increase indications or actively reduce prices, benign competition in the industry is beneficial to patients. In the 2019 national health insurance negotiation, the Tereprimab of Junshi Biology, the Sindhilimab of Cinda Biology and two imported drugs were shortlisted in this round of health insurance negotiation. Among them, Cinda Bio's Cindelimab was actively entered into the new version of the national medical insurance directory at a price of 2843 yuan (10ml: 100mg/bottle), which was 63.73% lower than the price of 7838 yuan (10ml: 100mg/bottle) at the time of listing.
According to the officially announced plan, patients who have been treated with Baekje Shenzhou Tirelizumab injection for a long time can get a full year of drug treatment by paying only five courses of treatment, with the minimum annual treatment cost of 106900 yuan. Baekje China said publicly that "the negotiation company will also participate in the future, and the PD-1 price is expected to go down".
As for the plan of whether to carry out subsequent medical insurance negotiations, the relevant person in charge of Junshi Biology said that Junshi Biology will also actively participate in the adjustment of the national medical insurance catalogue with the original intention of "providing better curative effect and more affordable treatment options for patients" this year, and strive to enter the catalogue at a reasonable price to benefit more patients through various ways.
Li Xu said that the annual treatment cost of Cinda Bio Sindilimab after the price reduction was about 96000 yuan, and the annual treatment cost of PD-1 drug from major manufacturers was expected to fall to less than 80000 yuan before it was expected to enter the medical insurance list this year. According to Zhao Heng, founder of medical strategy consulting company Latitude Health, according to the trend in the pharmaceutical field, the market competition of PD-1 products is becoming more intense, and the product price will also decline with the medical insurance policy. It is understood that since this year, enterprises including China Biopharmaceutical and Beida Pharmaceutical have successively entered the PD-1 market.
(Editor in charge: Shan Zixuan (intern), Wang Zhen)





















