Antibody drug exploitability analysis (drug producibility analysis) is a key strategy for evaluating the development prospects of candidate antibody drugs. This analysis is based on the comprehensive evaluation of many aspects of candidate antibodies, including biological activity, pharmacology, pharmacokinetics, safety, and preparation feasibility. Through systematic development analysis, candidate antibodies with potential clinical application prospects and commercial value are screened. This strategy is of great significance for narrowing the scope of candidate antibody drugs, reducing the development risk and saving time and cost. At the same time, this analysis strategy is also helpful to predict the performance of drugs in clinical trials and marketing stages, and provide guidance for clinical transformation and commercial development.
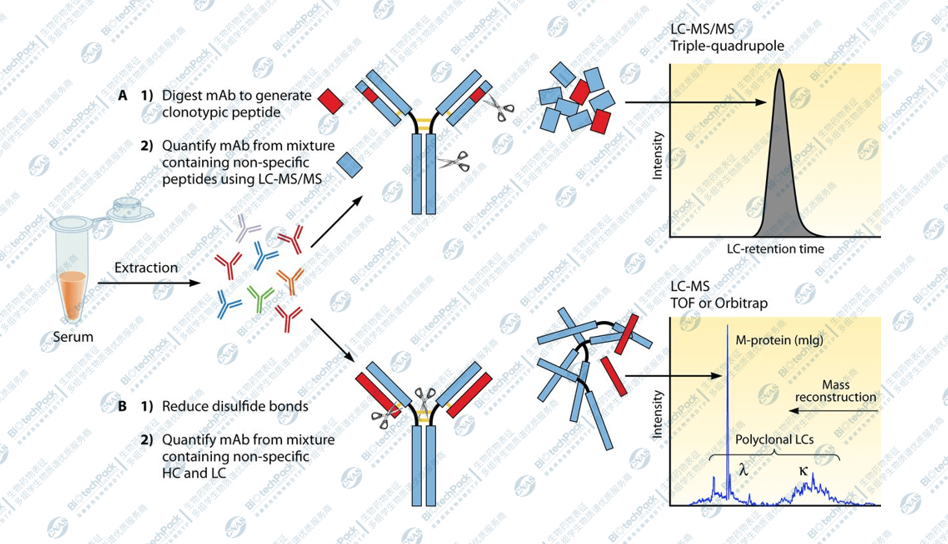
Antibody drug analysis based on LC-MS
Mass spectrometry plays a key role in the exploitability analysis of antibody drugs :
• Structural identification of antibody drugs: The primary structure of antibody drugs can be accurately analyzed by mass spectrometry. Through peptide fingerprint and tandem mass spectrometry, the amino acid sequence can be accurately identified, and some key post-translational modifications such as glycosylation and phosphorylation can be detected.
• Post translation modification analysis: The function and stability of many antibody drugs are related to their post-translational modifications. Mass spectrometry can be used to identify and quantitatively analyze these modifications to understand their impact on drug properties.
• Antibody assembly and heteropolymer analysis: Mass spectrometry can be used to analyze the assembly state of antibodies and the formation of heteropolymers, which is crucial for evaluating the stability and biological activity of drugs.
• Molecular weight analysis: Mass spectrometry can be used to confirm the molecular weight and isotope pattern of antibody drugs, which helps to ensure the quality and consistency of drugs.
Biotech adopt CNAS/ISO9001 dual quality system certification , based on Thermo's Orbitrap Fusion Lumos mass spectrometer combined with Nano LC nanoliter chromatography technology, Provide you with Antibody drug exploitability analysis (drug performance analysis) Service, which can comprehensively characterize candidate antibody proteins and help the development of new antibody drugs. Welcome to free consultation!
Project report in Chinese/English
In the technical report, Patek will provide you with a detailed technical report in both Chinese and English, including:
1. Experiment steps (Chinese and English)
2. Relevant mass spectrum parameters (Chinese and English)
3. Details of antibody drug exploitability analysis/drug completion analysis
4. Mass spectrum picture
5. Original data
One stop service of antibody drug exploitability analysis/drug producibility analysis
You only need to place an order - send samples
One stop service of BTP: sample processing - computer analysis - data analysis - project report
Related services
Analysis of Antibody Coupled Drugs (ADC)
Analysis of C-terminal K deletion ratio of antibody
Biopharmaceutical analysis
Antibody sequencing
De novo sequencing of monoclonal antibodies