-
Fujian Province's commemoration of "March 15" International Consumer Rights Day series activities in Rong -
58 minutes! Fuxing EMU Test Run in Fuzhou Xiamen High Speed Railway -
Meet Xi Jinping | He said we are peers -
Fujian has "number" | digitalization makes "poetry and distance" within reach -
Li Shulei Attends the Opening Ceremony of the 6th Digital China Construction Summit in Fuzhou -
The source of investigation and research flows in Fujian
-
The first meal of school for escort, we prepare "food" -
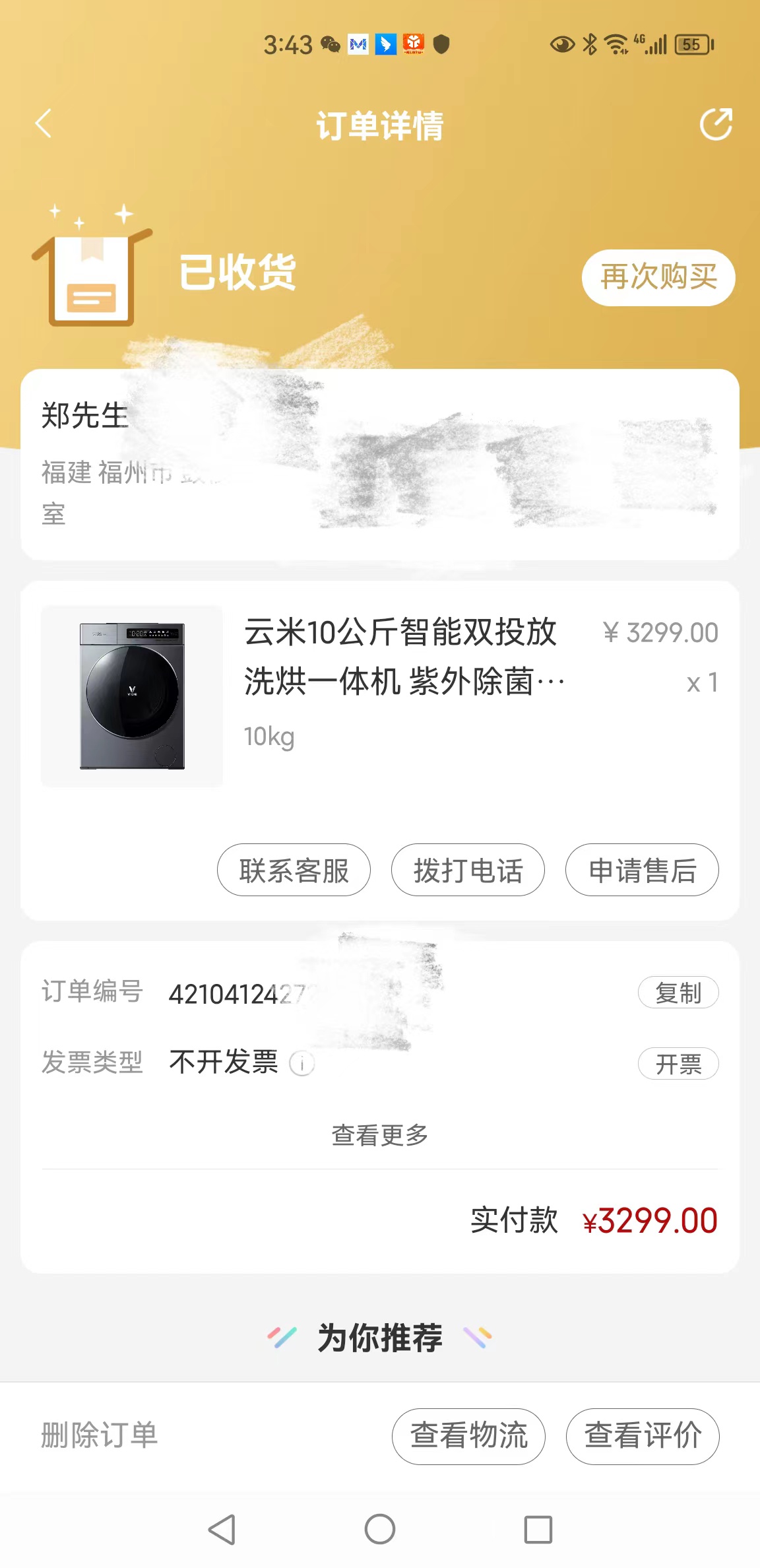
Online shopping is easy to place orders, and after-sales rights protection is difficult -- Yunmi whole house Internet appliances are accused -
Businesses procrastinate and do not want to perform the contract, consumers complain and protect their rights -
The Private Education Agreement is suspected of infringement -
Construction waste on the roof of Fuzhou Hongda Building threatens pedestrian safety -
Liquor sellers who sell infringing liquor were fined 22000 -
Refusal of winning the lottery, assisted by the consumer insurance center -
A manufacturer of unqualified sphygmomanometers in Xiamen was fined 10000 yuan -
Xianyou: False advertising merchants will be fined 4000 -
Jin'an: Mediate two complaints to help prevent and control the epidemic
-

Online shopping is easy to place orders, but after-sales rights protection is difficult - Yunmi Whole House Mutual -

The fourth member representative meeting of Fujian Charity Federation was held -

This year, Xi Jinping's anti epidemic "cloud diplomacy" highlighted major countries -

A special commemorative summit brought more than 2 billion people -

Why does Xi Jinping emphasize "the real -

Initial impression | witness the history of "entering the world hammer" -

Xi Jinping's trip to Tibet - the first stop to the Yarlung Zangbo River and -

”Special Column "Market Supervisor Presents Centennial Party Building"
-
one Gloss: carry out the work of "double random, one open" -
two Double 11 Dynamic Fuqing -
three Double Eleven Dynamic Longyan -
four Double 11 dynamic Nanjing -
five Favourites of Fujian people: mobile phones and rice -
six The deposit is not refundable, and consumers are trapped in "Double 11" -
seven Shao Wu Cracks Down on Illegal Medical Beauty -
eight Three cases of illegal addition of borax were investigated in Zhangping -
nine "Unattended store" group standard release -
ten Mawei checks product quality and safety hazards




