 Flu season is almost upon us. By now, you and your customers may have an idea of what’s to come, based on howthe flu season has already played out in other parts of the world. Last year, however,despite forewarningthat a vicious flu season was likely headed our way, the United States and European countries were caught off guard with a“Perfect Storm”of challenges for the influenza season. Flu test and antiviral medication shortages combined with limited effectiveness of the vaccine and a the aggressive H3N2 strain made for challenging times for both physicians and patients. Now, physician offices are getting ready to stock flu tests and other supplies in preparation for this year’s flu season. What do they need to know before they choose a flu test?
Flu season is almost upon us. By now, you and your customers may have an idea of what’s to come, based on howthe flu season has already played out in other parts of the world. Last year, however,despite forewarningthat a vicious flu season was likely headed our way, the United States and European countries were caught off guard with a“Perfect Storm”of challenges for the influenza season. Flu test and antiviral medication shortages combined with limited effectiveness of the vaccine and a the aggressive H3N2 strain made for challenging times for both physicians and patients. Now, physician offices are getting ready to stock flu tests and other supplies in preparation for this year’s flu season. What do they need to know before they choose a flu test?
Flu Diagnostic Challenges
Quite frankly, if a physician’s office is just gearing up to stock up, they’re already behind. For several reasons (inventory space concerns,”flu fatigue”, and the mindset that this year cannot be as worse as last) many clinicians may not think to buy early or stock up on flu tests and supplies. Additionally, at the end of last year’s season, some physicians returned surplus stock to clean up storage space. This is potentially short-sighted and could lead to problems once flu season gets going.
When physician offices need guidance, distributors should be consultative about planning ahead. They may be aware of complications, like when anFDA flu reclass led to shortages because some products were discontinuedin 2017/2018. This is why it is very important for manufacturers and distributors to work closely together to appropriately manage demand.
Planning Ahead
So much of flu season is a guessing game, especially when trying to plan for it. The World Health Organization (WHO), the Center for Disease Control (CDC), and the Food and Drug Administration (FDA) weighed in theirrecommendation to which strains should be included in the upcoming flu vaccinewell before the season gets started. Their prediction on what strains should be included will dictate how effective the vaccine will be. How severe the flu season is difficult to predict ahead of time, andthe key indicators used by the WHO, CDC, and others can only be obtained after flu season begins!
So, what can clinicians and distributors do to prepare?
First, conducting a business assessment of what the clinicians experienced last year and looking at historical data can help dictate a plan to ensure they procure enough product.
Second, weigh the pros and cons of the tests they are currently using and tests they might be considering. Clinicians should consider the following:
- Performance—Is test sensitivity and specificity the most critical?
- Volume—How many tests does your facility perform during an average flu season?
- Ease of Use – how simple is the test to perform?
- CLIA Complexity – Does the facility require Waived Complexity (Thesesimple and accurate testsare exempt from CLIA health and safety standards) or Moderate Complexity (Thesetests are more complexand have requirements for quality control, quality assurance, and more) Tests.
- Results reporting—Is it important for your facility to have an instrument-based result or visual read result, or is either one acceptable?
- Sample type—Does the test need to allow for multiple sample types (nasal, nasopharyngeal, aspirate/wash, and/or viral transport media (VTM))?
- Connectivity—Is it important for the testing device to be able to transmit the results electronically?
- Cost of the test
- The time to result
- Does the test require confirmation testing for negative results?
What’s Out There?
There are several types of flu tests available on the market.Rapid molecular testsdetect the genetic material of the virus and typically produce results in 30 minutes or less. These are considered to be more accurate than rapid influenza diagnostic tests (RIDTs). RIDTs arelateral flow immunochemical membrane teststhat can be either read visually or by an instrument and are intended to detect the presence (or absence) of a target antigen in 15 minutes or less.
FLU DIAGNOSTIC OPTIONS- ACCURACY, TIME AND COST COMPARISON
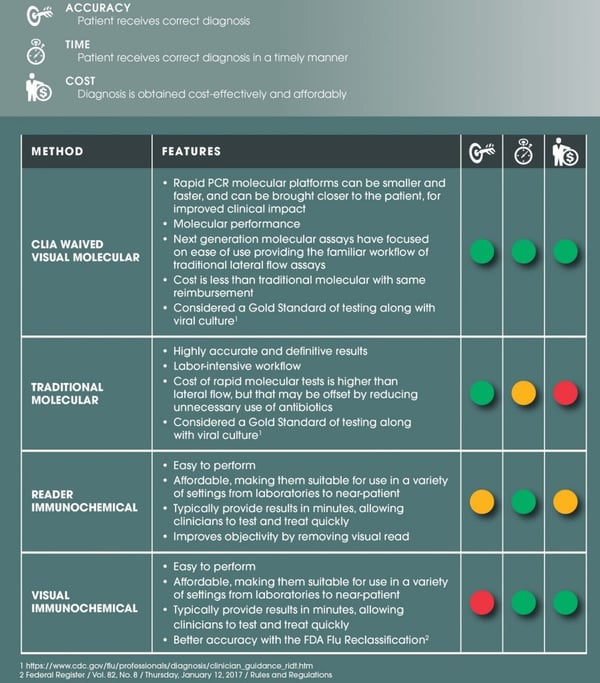
Depending on what characteristics (from above) are important to the clinician will help determine what type of test is a good fit for the facility. The manufacturer and distribution representative would be able to help guide the clinician to a product that is just right for them.
It should be noted that no flu test provides 100% accuracy. Results depend on the type of test used, the strain of virus, the integrity of the sample,even the age of the patient! It is very important when bringing on any test to review any limitations of the test, such as strain detection, any patient age limitations and performance data, along withsample collectionand handling best practices and make sure that all staff is trained to provide the best in class testing.
The Importance of Testing
Due to the known performance issues surrounding the RIDTs, some physicians may argue that it’s not necessary to administer a flu test in order to diagnose and treat.
Testing does not usually change how a patient will be treated for a flu diagnosis, so why bother? It turns out there are several reasons. Empirical treatment has a disadvantage in that many more patients are receiving treatment than actually have the flu giving rise to a possible antiviral shortagethe likes of which we saw last yearand possibly delaying the right treatment for another health issue. Testing patients provides valuable information to the clinician that can enable them to rule out other illnesses, helps determine a more direct therapy plan, and reduces the risk of unnecessary antiviral or antibiotics therapy, while increasing the chances that the patient will receive anti-viral therapy early when it is most effective. Testing will also helpdetermine whether an outbreak of flu is occurring. Since the implementation of rapid molecular tests and the drive by the FDA reclassification to have better RIDTs into the market clinicians can have the opportunity to utilize a highly accurate test to be more confident in the results to drive direct therapy.
How Sekisui Diagnostics Can Help
Sekisui Diagnostics offers two flu tests to help clinicians master the art of influenza testing.
The CLIA-WaivedSilaris™ Influenza A&B Testis a molecular diagnostic test utilizing polymerase chain reaction (PCR) technology providing accurate results for early diagnosis and proper management of influenza.
The CLIA-WaivedOSOM® Ultra Flu A & B Testis a FDA Class II compliant in-vitro rapid qualitative test that detects influenza type A and type B nucleoprotein antigens directly from nasal swab, nasopharyngeal swab, and nasopharyngeal aspirate/wash specimens obtained from patients with signs and symptoms of respiratory infection.
The 5 “Ps”
 In the end, it comes down to the 5 “Ps” – Proper Preparation Prevents Poor Performance!
In the end, it comes down to the 5 “Ps” – Proper Preparation Prevents Poor Performance!
Don’t be afraid to keep stock of flu tests all year round. Understand new options—with the fear of changing strains, new technologies are more important than ever. You have choices! Manufacturers are trying to provide new technologies to keep up with flu diagnostic challenges.





